Plasma is a unique state of matter in which gases can become ionized, consisting of electrons, ions, and neutral atoms or molecules (e.g., anions, cations, radicals, and neutrals; in ground and excited states). Low-temperature plasmas often exhibit non-equilibrium conditions, with the electron temperature vastly exceeding the gas temperature (often referred to as non-thermal plasmas). Because of these unique conditions, it can drive chemical reactions that are otherwise challenging, or even impossible, to achieve with conventional thermal methods. Low-temperature reactive plasmas represent a key driver for technologies of socioeconomic transformations. For example, plasmas are indispensable in the manufacturing of solar panels and microelectronic. Our ability to model and optimize these plasmas relies on precise knowledge of reactive species’ concentrations, temperatures, and reaction kinetics.
Absorption spectroscopy is a well-established tool for analysing gaseous samples and quantifying molecular concentrations. Each molecular species in the plasma will then have a unique absorption spectrum reflecting its number density.
Plasma-assisted Nitrocarburizing
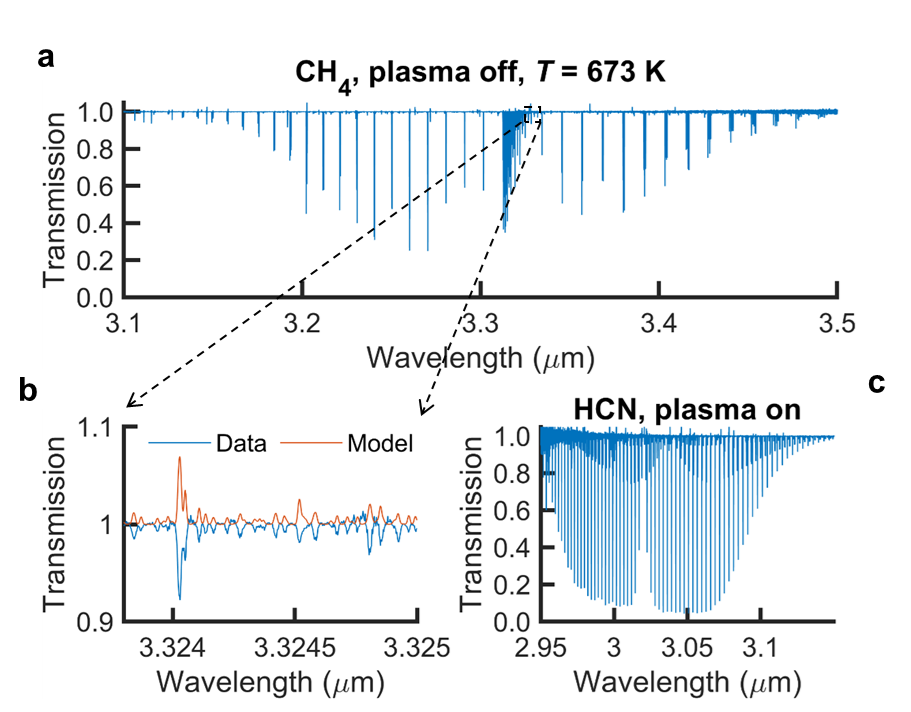
Plasma-assisted nitrocarburizing (PNC) is a prime example of low-temperature molecular plasma applications. PNC is used to enhance the hardness of stainless steel and metals (in general), and it is considered as an eco-friendly alternative to conventional gas nitrocarburizing that is more energy-intensive and consumes considerable amount of materials. In PNC, reactive nitrogen- and carbon-containing species that are generated in the plasma, diffuse into metal surfaces and enhance their hardness and fatigue strength. This process is in high demand in industries such as automotive and aerospace.
We utilize an optical frequency comb light source in the mid-infrared spectral region, along with broadband detection methods such as Fourier transform spectroscopy and cross-dispersive virtually imaged phased array (VIPA) spectroscopy, to quantify the molecular composition of PNC. Our particular focus lies in understanding the complex chemistry of hydrogen cyanide (HCN) in plasmas, which is one of the key species produced, and it is believed to affect plasma chemistry and, consequently, the quality of nitrocarburized tools.
Selected Publications:
[1] Sadiek I, Fleisher AJ, Hayden J, Hugi A, Lang N, van Helden JPH, High-Resolution Dual-Comb Spectroscopy at 9.5 μm for Plasma-Assisted Ammonia Production. CLEO: Applications and Technology, 2023, ATh1K.2.
[2] Sadiek I, Fleisher AJ, Lang N, van Helden JP, Absolute Abundance Measurements of Molecular Species in a Plasma Reactor with Non-Uniform Temperature using Optical Frequency Comb Fourier Transform Spectroscopy, CLEO: Applications and Technology, 2023, ATh1K. 3.