We investigate the potential energy surface (PES) and the unimolecular dissociation pathways for molecular systems of atmospheric and industrial importance.
For example, we explored the unimolecular decomposition of bromoacetaldehyde (BrCH2CHO) – a significant stable brominated organic intermediate in the bromine-ethylene addition reaction during arctic bromine explosion events. We performed high-level quantum chemical calculations, and Rice−Ramsperger−Kassel−Marcus (RRKM)/Master equation analysis under photolytic and thermal conditions. We found that there are 14 possible decomposition channels for BrCH2CHO on a PES with eight wells. Assuming a singlet ground-state potential energy surface dominated photodynamics, we studied primary photodissociation yields of BrCH2CHO under various collision energy transfer conditions. At ground level, under tropospheric actinic flux and atmospheric pressure, depending on collisional energy transfer (150 cm−1 < ⟨ΔEdown⟩ < 450 cm−1), 33−78% of BrCH2CHO directly photodissociates at 320 nm. This is notably higher than acetaldehyde (14%) due to favorable Br and HBr-forming dissociation channels. Photodissociation product branching fractions are relatively independent of collisional energy transfer. At ⟨ΔEdown⟩ = 300 cm−1 and 320 nm excitation, 27% involves C−C bond fission, 11% C−Br bond fission, 7% HBr elimination, and < 2% each for consecutive O−Br fission and brominated vinyl alcohol photo-tautomerization, notably different from acetaldehyde’s photodissociation.
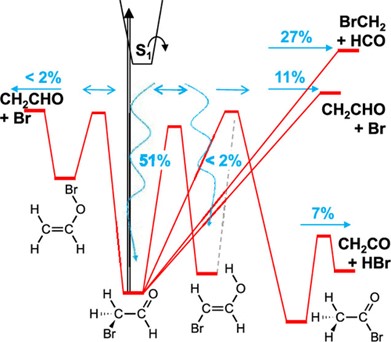
Major photodissociation product branching fractions of acetaldehyde at ⟨ΔEdown⟩ = 300 cm−1 and 320 nm excitation: 27% involves C−C bond fission, 11% C−Br bond fission, 7% HBr elimination, and < 2% each for consecutive O−Br fission and brominated vinyl alcohol photo-tautomerization.
Selected Publications:
[1] Sadiek I, Friedrichs G, Sakai Y, Ab initio and RRKM/master equation analysis of the photolysis and thermal unimolecular decomposition of bromoacetaldehyde. J. Phys. Chem. A. 2021;37:8282-93.
[2] Elshakre M, Sadiek I. A DFT study of the dissociation, ionization, and UV/Visible spectra of methyl hypobromite. Comput. Theo. Chem. 2016;1088:32-43.